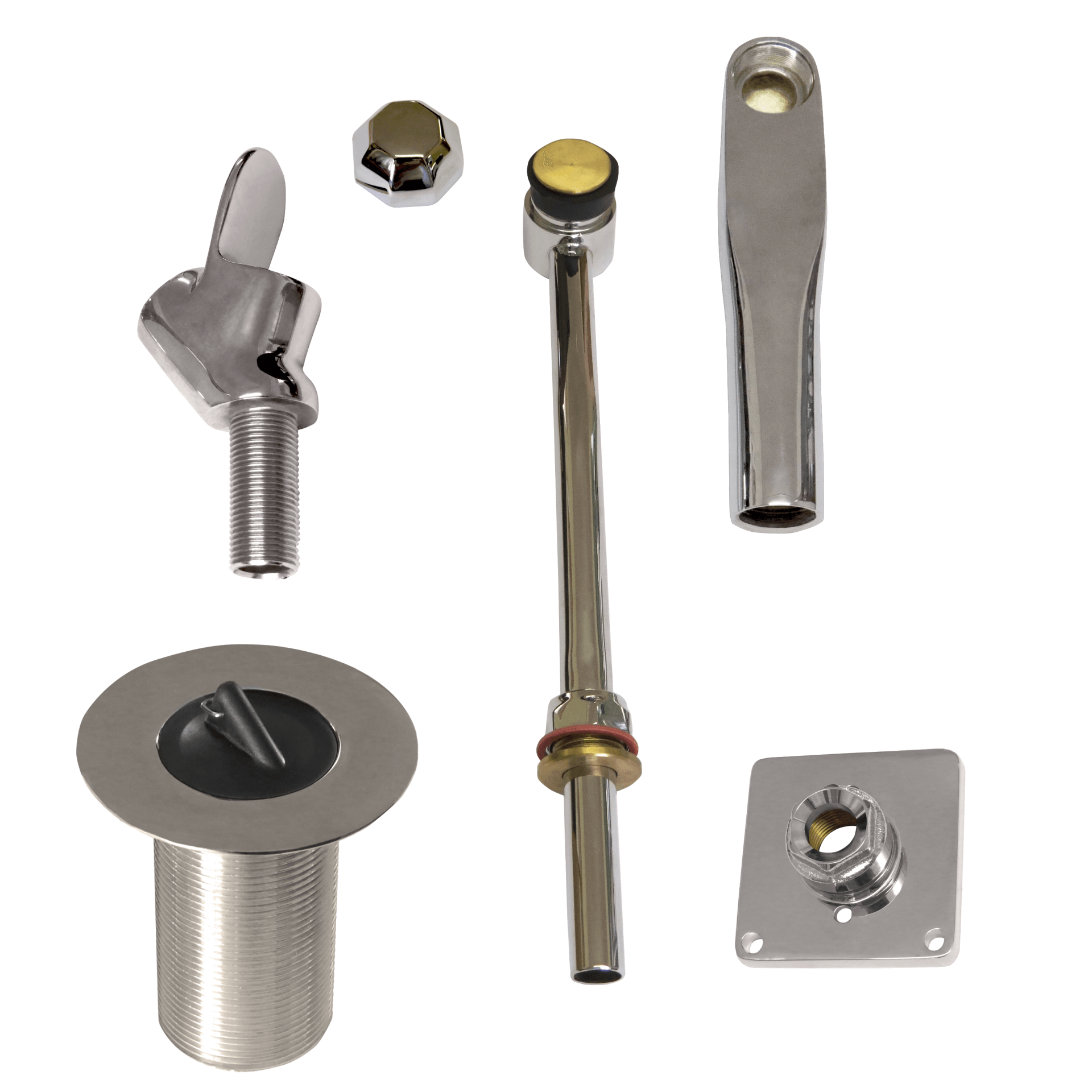
DZR BRASS
Dezincification of brass is a critical aspect of the fitness for purpose (quality) of plumbers fitting that is in contact with water. The risk and rate of dezincification increases with water hardness and the acidity or alkalinity of water away from a PH of 7. Dezincification is the name given to the corrosion of brass. Dezincification resistant brass, or DZR brass, (something designed CR or DR) is the name given to brass that has exceptional resistance to this corrosion. The resistance is imparted by the adherence to SABS specifications for chemical composition and careful process controls. In order to be called DZR brass each batch must pass an ISO 6509 dezincification resistance laboratory test.
In South Africa the use of DZR brass for components of brass plumber’s fittings that are in contact with water is national law. Building inspectors are being trained to demand the removal of plumber’s brassware that does not conform to statutory law.
BRASS
Brass was invented around 3000 BC in the Middle East and records exist of its use until 1400 BC – 1000 BC when it fell into disuse until around 500 BC. The alloy reached Europe in the first and second century AD and became an established industry in the 15th and 16th century. Brass is the name given to an alloy of copper and zinc. Copper is a noble but malleable metal, that is, it is a non-reactive, stable material and is therefore ideal for use in a corrosive environment i.e. plumbers fittings. Its drawback is its malleability, it readily deforms and threads cut are not strong. Copper is easily worked when cold but a difficult material when in a molten state in the foundry. Zinc is a reactive but strong metal. On its own it would not be suitable in a corrosive environment such as a plumber fitting. Zinc is difficult to work when cold but very easy to cast. When alloyed together in proportions of around 58% copper to 40% zinc the resultant alloy, brass, is suitable for plumbers fittings with regard to strength (from the zinc) and corrosion resistance (from the copper). If the copper percentage is lowered and thereby the zinc raised the brass becomes easier to cast and work with. This increases brass factory output and the lower cost of zinc further increases factory profitability, but however it also increases the re-activity of the brass alloy, making it more susceptible to corrosion. If the copper percentage is increased the opposite happens. The material becomes more difficult to cast, lowering factory output. Factory tooling wears out much more quickly raising overheads and the high cost of copper further increases costs. The balance of material in the last 2% in a typical 58% copper to 40% zinc, non DZR brass, is taken up by industry-accepted ingredients to impart mach inability qualities, and impurities. These additives and impurities act as zinc equivalents (except for nickel which is a copper equivalent). This means that their concentration adds to the zinc percentage that, albeit helping with factory production and costs, makes the final product, the tap that you sell more susceptible to corrosion. Some of these additives and impurities can have a significant impact on the brass. For example look at this table
Zinc equivalents (ZnE)
| Element | Zinc value |
|---|---|
| Silicon (Si) | 10 |
| Aluminium (Al) | 6 |
| Tin (Sn) | 2 (but is a grain refiner) |
| Lead (Pb) | 1 (not soluble) |
| Iron (Fe) | 0.9 (but is a grain refiner) |
| Manganese (Mn) | 0.5 |
| Nickel (Ni) | Copper value 1.2 |
This table shows that the effect of silicon for example in a brass will have the same impact as 10 times its weight of zinc. Clearly impurities and additives have to be very carefully monitored and controlled to maintain resistance to corrosion. COBRA DZR brass has a copper content in excess of 62% and has stringent control on impurity levels and additive quantities.
Dezincification of Brass
Dezincification of brass is an example of ‘de-alloying’ in which one of the constituents of an alloy is preferentially removed by corrosion. In the case of brass this constituent is zinc, hence the name dezincification. Copper and zinc are not found as separate constituents in brass, the two elements go into solid solution, that is, they completely mix together at every atomic level. However, as part of the solidification process of an alloy of copper and zinc at around the 60% 40% mixture, areas of the body of brass become slightly higher in copper concentration, this is called an ‘alpha phase’. Other areas become slightly higher in zinc concentration, these areas are called ‘beta phase’. See attached a thermal equilibrium diagram showing the alpha and beta phase distribution in relationship to regard to copper/zinc/temperature co-ordinates. The process by which dezincification occurs is that instead of the zinc being selectively leached out in corrosive conditions, the whole body of the brass passes into solution. The difference in electrical potential between alpha and beta phases in the brass bring about a galvanic action which electroplates only the lost copper ions back into the brass fitting at the site of the corrosion attack. Due to this electroplating of the copper ions dezincified brass will retain the original shape and dimensions of the metal component before corrosion but the copper residue is porous and has very little strength. If the dezincified brass fitting is a tap, the tap will fail and water will leak to the extent of the failure.
Dezincification was first recognised as a serious problem in brass tubes used in ships condensers before about 1920. It was stated that “condenseritis” i.e. dezincification of condenser tubes had more effect than the German navy in putting HM ships out of action in the first world war. Research on the problem by G D Bengough and R May established incorporating dezincification-inhibiting elements could prevent that dezincification. This work resulted in the formulation of dezincification resistant Admiralty brasses, which in turn have been written into British Standards and are used by Cobra in all brass components that are in contact with water.
Dezincification of brass plumber’s fittings in some districts was first recognized in England in the late 1950’s and in South Africa in the 1960’s. The recognition in South Africa was brought about by the fact that the dead of night water flow into the major residential areas of Johannesburg was some 70% of the peak usage flow. This was later found to have been caused by leakage of underground water pipes due to the failure of dezincified brass fittings. This was a type of dezincification now called ‘meringue dezincification’, in which the zinc passing into solution from the brass forms very bulky hollow mounds of corrosion product, which block the fitting. It attacks the beta phase preferentially but will eventually spread.
Recognition
Dezincification may show itself as dull red spots developing on the surface of brass after periods of exposure to urban or industrial atmospheres. These do not usually represent any significant loss of strength in the component concerned but since they are more than simply superficial they cannot be removed by the cleaning and polishing procedures that would normally the brass to its original appearance. Dezincification in water fittings, taps and valves etc. can show itself in a variety of ways depending on the water composition and service conditions. Water may be seen seeping through the walls of fittings with an accompanying whitish deposit of zinc and lime scale on and around the leak site. In exposed fittings this would probably be the time when the corrosion and resultant water leak is noticed and attended to. On under wall or underground fittings dezincification takes on worse proportions as the dezincification could be occurring from both inside and outside of the fitting. The first small loss of water will probably be noticed until the time that flooding occurs due to gross breakage.
Conditions for dezincification Brass
There are a number of factors that will predispose brass to de-zincify.
- Water hardness and the acidity or alkalinity of water away from a PH of 7.
- Temperature. The higher the temperature the greater the risk
- Water flow. Less flow equals greater risk
- Polluted atmosphere
- Large brass grain size
- Sea or brackish water
- Corrosive soils such as acid peat, salt marsh, waterlogged clay, or ‘made up’ ground containing cinders
-
What DZR specification does EXIPRO follow?
EXIPRO DZR brass for casting ingot follows the specification of SA alloy code 9A which is a derivative of British Standard DZR brass composition CZ132.
In addition to this extra grain refining processes are exerted on the material in the foundry. There is a definite link between grain size and dezincification resistance that is documented within the industry and has been the subject of extensive study. The smaller the grain size the greater the resistance to corrosion.
EXIPRO brass is refined to the point that individual grains no longer exist and our brass is one homogenous mass. These refining processes are in addition to the composition requirements.
EXIPRO brass for pressing and turning follows the same specification as the casting material but with slight modifications to suit the machining processes to which it will be exerted.
In order to maintain impurity levels lower than can be expected from raw material brass suppliers
EXIPRO has built a smelting plant and we produce our own raw material brass ingot.
Each of our two brass foundries has its own on-site laboratory equipped with Fisons 3460 spark
emission spectrometers. The chemical composition of each production furnace is constantly monitored and kept within the written specification for 14 separate elements.
Each EXIPRO component has a sub-chemical composition specification, which allows for the best composition for a given component within the broader DZR specification. For example, one head part cover casts best at 0.25% aluminum and another similar item is best at 0.18%.
Composition control is maintained in mass production right down to these minuscule amounts.

Manufacturing & Exporting of Quality Brass Components
Industries EXIPRO served
| Agriculture | Architectural & Decorative | Construction |
|---|---|---|
| Fire Protection Products | Hydraulic Fluid Power | Plumbing |